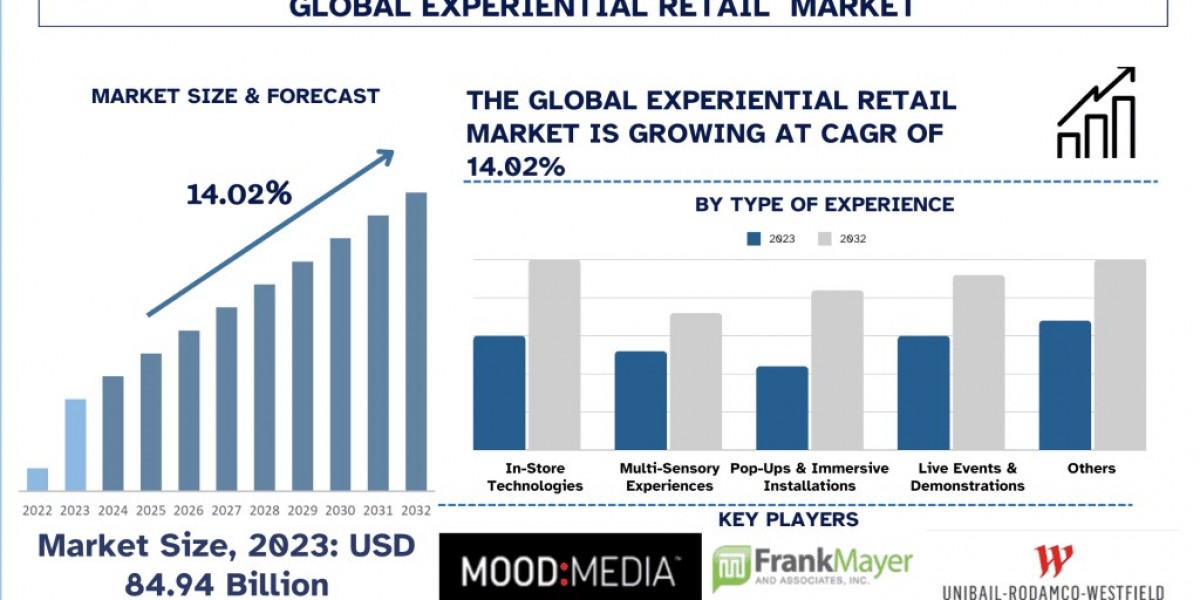
The U.S. small molecule CDMO market was valued at USD 26.81 billion in 2024 and is projected to reach USD 49.82 billion by 2034, growing at a CAGR of 6.4% between 2025 and 2034. This surge reflects the country’s increasing dependence on contract development and manufacturing services to meet the rising demand for innovative therapies, cost optimization, and faster drug development timelines. To explore the full landscape, readers can refer to U.S. small molecule CDMO market.
Market Overview
The United States represents one of the most dynamic pharmaceutical markets in the world, characterized by high levels of drug innovation, clinical research activity, and regulatory rigor. While large pharmaceutical firms continue to dominate the innovation pipeline, contract development and manufacturing organizations (CDMOs) are playing an increasingly central role in ensuring cost efficiency, scalability, and access to specialized technologies.
Small molecule drugs remain the backbone of the U.S. pharmaceutical sector, accounting for a majority of prescriptions. CDMOs are bridging the gap between discovery and commercialization by providing flexible solutions such as formulation development, active pharmaceutical ingredient (API) production, analytical testing, and regulatory compliance support.
LSI Keywords: contract manufacturing services, pharmaceutical outsourcing, U.S. drug development, clinical trial support.
Market Segmentation
The U.S. small molecule CDMO industry can be segmented into several categories:
- By Service Type
- Development Services: pre-clinical research, formulation, and process optimization.
- Manufacturing Services: large-scale API production, final dosage form (FDF) manufacturing, and packaging.
- Regulatory & Analytical Support: ensuring compliance with FDA and international regulations.
- By Therapeutic Area
- Oncology – the fastest-growing area, given rising cancer prevalence.
- Cardiovascular Disorders – significant demand due to the U.S.’s aging population.
- Neurology & CNS Disorders – increased outsourcing for complex central nervous system drugs.
- Others – metabolic and infectious diseases.
- By End-User
- Large Pharmaceutical Companies – driving the majority of outsourcing demand.
- Biotechnology Firms – partnering with CDMOs for speed-to-market.
- Generic Manufacturers – outsourcing production to control costs.
Regional Analysis within the U.S.
Unlike global markets, the U.S. small molecule CDMO sector shows regional disparities:
- Northeast U.S. (Boston, New Jersey, New York): hosts many pharmaceutical headquarters and academic research centers. CDMOs here specialize in early-phase development and regulatory consulting.
- Midwest (Chicago, Minnesota): manufacturing-focused hubs with API production facilities.
- West Coast (California): driven by biotech startups and innovation, with a strong emphasis on R&D outsourcing.
- Southern States (North Carolina, Texas): emerging CDMO clusters due to lower operational costs and proximity to pharmaceutical clients.
Key Drivers
Several factors are fueling the rapid expansion of the U.S. CDMO industry:
- Pharma Innovation: High investment in research pipelines requires scalable partners.
- Cost Efficiency: Outsourcing helps reduce capital expenditure for drug firms.
- Regulatory Complexity: CDMOs with FDA expertise are in high demand.
- Biotech Growth: Emerging biotech startups rely heavily on CDMOs for specialized manufacturing.
- Global Supply Chain Risks: Onshoring trends are strengthening U.S.-based CDMOs.
Key Companies in the U.S. CDMO Market
Leading players include:
- Catalent, Inc.
- Cambrex Corporation
- Thermo Fisher Scientific (Patheon)
- Alcami Corporation
- Lonza (U.S. operations)
- Cambrex Charles City Facility
- Piramal Pharma Solutions (U.S. arm)
- Alcami Holdings
- PCI Pharma Services
These companies are investing in advanced manufacturing technologies, expanding FDA-compliant facilities, and forming strategic alliances with pharmaceutical innovators.
Conclusion
The U.S. small molecule CDMO industry will continue to thrive, benefiting from outsourcing trends, a strong pharmaceutical pipeline, and government emphasis on domestic manufacturing. By 2034, the market is projected to reach USD 49.82 billion, underlining its role as a strategic pillar of the American pharmaceutical supply chain. For official press insights and more details, please visit U.S. small molecule CDMO
More Trending Latest Reports By Polaris Market Research:
Distributed Antenna System (DAS) Market
Industrial and Commercial LED Lighting Market
Connected Medical Devices Market
High Performance Composites Market
Stadium and Arena Management Solution Market