Polaris Market Research announces the release of its latest research report titled, Laboratory Developed Tests Market. The report offers an in-depth analysis of the global market. It outlines current market conditions and future growth potential over the forecast period. It includes comprehensive data-backed insights into emerging trends, innovation pipelines, and competitive movements to help stakeholders understand key shifts driving global market evolution. Through extensive primary and secondary research, the report quantifies market performance and provides a holistic view of demand patterns, pricing dynamics, and regional developments.
Market Stats
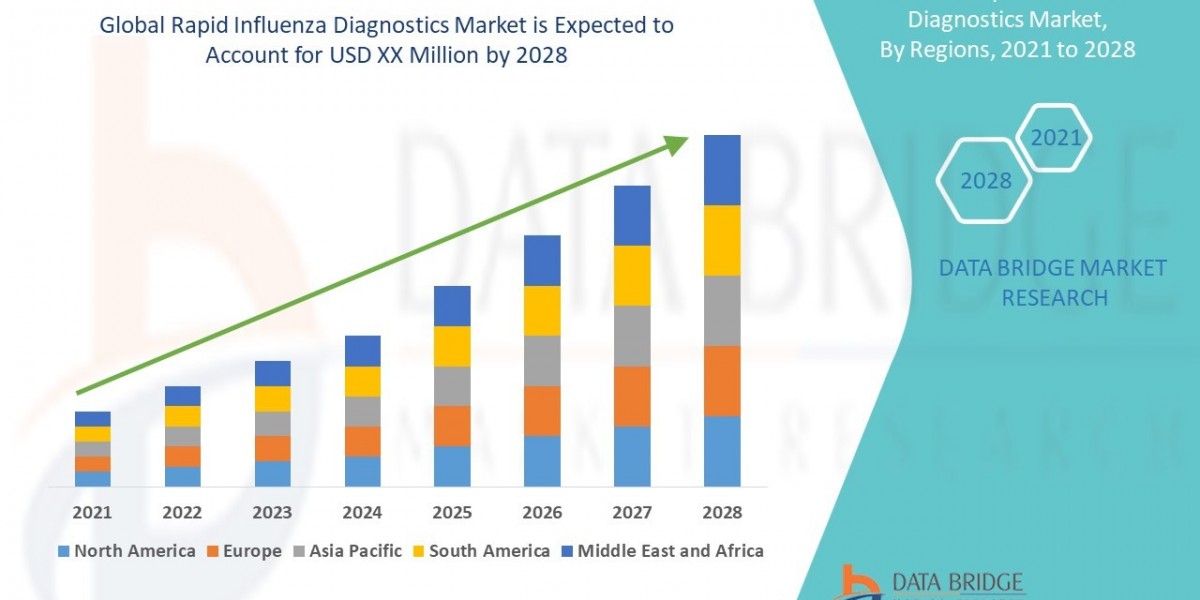
Global Laboratory Developed Tests Market size and share is currently valued at USD 12.21 billion in 2023 and is anticipated to generate an estimated revenue of USD 22.30 billion by 2032, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 6.9% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2024 - 2032
Market Definition
Laboratory Developed Tests (LDTs) are diagnostic tests that are designed, manufactured, and used within a single laboratory. Unlike commercially distributed in vitro diagnostic (IVD) kits, LDTs are not mass-produced but are tailored to meet specific patient or clinical needs, often in areas where no commercial tests exist. These tests are critical in specialized fields such as oncology, genetic testing, infectious diseases, and rare conditions, where rapid innovation and customization are essential. LDTs play a vital role in precision medicine by enabling laboratories to adapt to emerging diseases, mutations, and personalized treatment plans. Although primarily regulated under the Clinical Laboratory Improvement Amendments (CLIA) in the United States, ongoing discussions aim to bring more comprehensive oversight due to their growing complexity and usage. The market is supported by advanced molecular diagnostics, next-generation sequencing (NGS), and evolving biomarker discoveries, all contributing to the expansion and innovation of LDT applications across healthcare systems globally.
Market Dynamics
The report analyzes several factors that are shaping the Laboratory Developed Tests market landscape:
Technological Advancements
The report thoroughly examines how technological innovations are transforming the Laboratory Developed Tests market landscape. It explores how the integration of next-gen technologies is accelerating solution development cycles and broadening the range of practical applications. The study emphasizes the importance of these innovations in enabling market participants to differentiate their offerings and meet evolving customer demands.
Regulatory Push and Sustainability Goals
Another major driver identified in the report is the influence of regulatory frameworks and increasing emphasis on sustainability. Governments globally are introducing stricter mandates concerning compliance, safety standards, emissions control, and environmental impact. The report provides a detailed analysis of how these regulatory changes are accelerating market growth. The study explores how these sustainability imperatives are shaping solution development and investment priorities.
Browse Full Insights:
https://www.polarismarketresearch.com/industry-analysis/laboratory-developed-tests-market
Competitive Landscape
The report includes a detailed assessment of the competitive landscape of the market. It highlights the major market participants, their strategic initiatives, and recent developments. Company profiles feature data on product portfolios, R&D activities, regional presence, and partnerships. Special attention is given to innovation strategies, mergers and acquisitions, and new product launches that are influencing market direction. The report also discusses how emerging players are entering the market with disruptive technologies, contributing to increased competition and faster innovation cycles. An evaluation of pricing strategies, channel dynamics, and brand positioning is also provided in the study.
A few of the key market players are:
- 23andMe, Inc.
- Abbott
- F. Hoffmann-La Roche Ltd.
- Guardant Health
- Illumina, Inc.
- NeoGenomics Laboratories.
- QIAGEN
- Quest Diagnostics Incorporated.
- Siemens Healthiness AG
Key Report Highlights
- Provides comprehensive market size estimates and growth forecasts for the global market.
- Offers a detailed analysis of current and emerging market dynamics
- Examines the impact of regulatory shifts and sustainability mandates on innovation and market adoption rates.
- Highlights key industry trends shaping Laboratory Developed Tests market landscape.
- Analyzes supply chain developments, pricing trends, and raw material availability affecting overall market performance.
- Identifies growth opportunities across developed and emerging markets, with focused insights on industry verticals that are experiencing accelerated adoption.
Conclusion
The Laboratory Developed Tests market is at a pivotal stage of development, marked by rapid technological evolution and growing cross-sector integration. The report captures the current state of the market and also anticipates the shifts that will define its trajectory in the coming years. By outlining the challenges, competitive strategies, and innovation trends shaping the landscape, it offers a well-rounded foundation for strategic planning.
More Trending Latest Reports By Polaris Market Research:
Intelligent Document Processing Market
Intelligent Document Processing Market
Patient Temperature Monitoring Market
Chemical Injection Skids Market